DIGITAL THERAPEUTICS - THE INCREASING REGULATORY SUPPORT HAS FACILITATED THE ESTABLISHMENT OF A STANDARD DEVELOPMENTAL PATHWAY FOR THESE SOLUTIONS
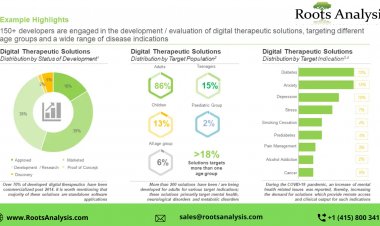
Digital therapeutics are clinically validated applications / software / online programs that have demonstrated the capability to facilitate positive outcomes when used in the prevention / treatment / management of diseases / clinical conditions.
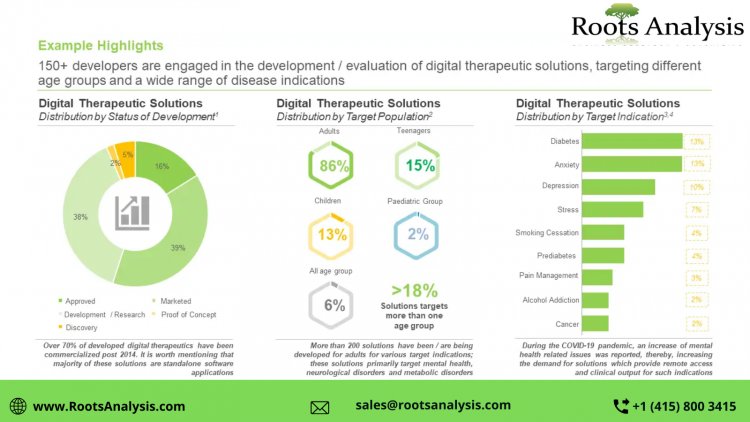
Digital Therapeutics, popularly known as DTx, represent a digital health solution which delivers medical interventions directly to the patients in order to treat, manage and prevent a disease. These therapeutics are designed to engage patients in personalized treatment or disease prevention programs, through mediating behavioral or psychological modifications, providing motivational support and inculcating healthy lifestyle changes.
Several organizations have undertaken diverse initiatives in the field of digital therapeutics to support its growth as a new frontier in the healthcare sector. A number of organizations focused on effectively monitoring and promoting the potential of digital therapeutics to be used as a part of strategies to improve the population health have also been established. These organizations include:
- Digital Therapeutics Alliance (DTA)
- Personal Connected Health (PCH) Alliance
- Centers for Disease Control and Prevention (CDC)
- Health Insurance Portability and Accountability Act (HIPAA)
- National Health Service (NHS)
- United States Food and Drug Administration (USFDA)
To request a sample copy / brochure of this report, please visit this
https://www.rootsanalysis.com/reports/208/request-sample.html
Given the current activity in this domain and the growing demand for such solutions, the digital therapeutics market is likely to grow at a healthy pace over the next decade. Traversing a digital therapeutic from the R&D stage to the market is a long process. The various developmental stages involved in this process have been discussed in detail in the following sections.
- Discovery and Preclinical Phase: The discovery phase involves the identification of a novel digital therapeutic intervention. At this stage, researchers publish their work in academic journals and continue to investigate the potential applications of their digital solutions in disease treatment / management.
- Clinical Trials and Validation: This phase involves the conduct of proper clinical trials to validate the claims made by a digital therapeutic solution provider, and to evaluate its potential in a real-world setting. It includes testing of the digital therapeutics software / hardware on a specific patient population. In case of clinical studies, health outcomes are measured and tracked through data driven insights provided by the software. Disease specific improvements (post application / implementation of the intervention) are also tracked to evaluate the performance of a product. There are multiple challenges associated with conducting clinical trials for digital therapeutics. Firstly, technologies are known to change rapidly and there is a very high probability for a software to undergo upgrades / improvements over the duration of a clinical trial. As a result, there are technical issues in storing and updating patient data. Secondly, digital interventions cannot be studied in a double-blind manner, because the investigator is always aware of whether a trial subject is in the control group or being treated with the intervention under evaluation. Finally, at present, there is less structure and guidelines available, and as a result meaningful and conclusive insights are difficult to be drawn from such trials.
- Negotiations with Insurance Providers / Payers: Post the successful completion of clinical studies, developer companies generally tend to avail reimbursement opportunities for their products in order to promote the use of their proprietary solutions and provide financial benefits to patients / consumers. As is the case with pharmacological interventions, reimbursement plans for these products can be achieved based on the outcomes of clinical trials and depending on the USFDA’s (or the concerned regulatory authority of a particular region) clearance. A number of health insurance providers, such as Medicare and Humana, are actively working to include digital therapeutics as a part of health insurance coverage plans for patients suffering from chronic diseases.
- Distribution and Marketing: The pharmaceutical and medical device distribution / marketing system is an established network with well-defined channels through which manufacturers can reach the end-users of their products. Product developers in this domain are presently looking to create a distribution network to sell their offerings in the market via both B2B (healthcare providers, regulators and payers) and B2C (customer) models.
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/digital-health-market/208.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
- AI-based Digital Pathology / AI Pathology Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com